Malaria causes about 1/4 billion new cases of disease and about 600,000 deaths every year. 3/4 of the deaths are children under 5 years old. The July 23, 2025, NEJM has a free full text article showing use of ivermectin to prevent malaria and potentially save a lot of lives. This should appeal to ivermectin proponents, since hundreds of thousands of lives could be saved by an effective intervention.
Malaria control and elimination is threatened by the spread of insecticide resistance and behavioral adaptation of vectors. Whether mass administration of ivermectin, a broad-spectrum antiparasitic drug that also kills mosquitoes feeding on treated persons, can reduce malaria transmission is unclear.
We conducted a cluster-randomized trial in Kwale, a county in coastal Kenya in which malaria is highly endemic and coverage and use of insecticide-treated nets are high. Clusters of household areas were randomly assigned in a 1:1 ratio to receive mass administration of ivermectin (400 μg per kilogram of body weight) or albendazole (400 mg, active control) once a month for 3 consecutive months at the beginning of the “short rains” season. Children 5 to 15 years of age were tested for malaria infection monthly for 6 months after the first round of treatment. The two primary outcomes were the cumulative incidence of malaria infection (assessed among children 5 to 15 years of age) and of adverse events (assessed among all eligible participants).
What about DDT?
Why can’t we just DDT the mosquitoes away? Surely, Dire Straits could have a Concert for DDT, singing that, I waaaant my DDT. The problem is resistance to DDT. Mosquitoes are not cooperating with plans to exterminate mosquitoes. Researchers have known about this DDT resistance for a while:
Insect resistance to dichlorodiphenyltrichloroethane (DDT) emerged in the 1940s, with the first conclusive study being conducted on the Culex molestus mosquitoes in 1947 in Italy. Insecticide resistance was also reported among Anopheles sacharovi mosquitoes in Greece in 1951 (23). In 1955, it was reported in the An. gambiae species in Nigeria (24). Thereafter, resistance has been reported in more than 500 insects, 50 of which transmit malaria parasites in humans (21, 25).
That simple solution is not an effective long-term solution, so the much more complex approach of medical intervention is currently needed. Genetically modified mosquitoes could be effective, but the technology is newish and opposition to this will be tremendous, even if there is abundant evidence of safety.
Take ivermectin
Ivermectin is an endectocide, a drug that is effective against endoparasites and ectoparasites, and is used in mass drug administration to treat onchocerciasis and lymphatic filariasis and to reduce their transmission. Through the Mectizan Donation Program established by Merck, more than 4.6 billion ivermectin treatments have been distributed safely since 1988 to populations in regions where malaria is endemic.6 Ivermectin can also kill malaria vectors that feed on treated persons.7 Thus, mass administration of ivermectin has been suggested as a potential strategy to reduce malaria transmission driven by mosquitoes that are either resistant to insecticides or evade them through behavioral changes.8 To address public health needs and guide the development of new malaria vector-control tools, the WHO produced the preferred product characteristics for endectocides in 2016 and updated it in 2022; one target characteristic that was considered to be of potential value to public health was a 20% reduction in the incidence of clinical malaria or infection.9,10
Our primary research question was whether mass administration of ivermectin, given in three monthly doses of 400 µg per kilogram of body weight at the start of the short rainy season (which typically spans from October through December), would result in a relevant reduction in malaria transmission in an eligible population and have an acceptable safety profile.11
From the Protocol supplementary material in PDF (page 125/266):
The study will be open label but outcome assessor-blinded. Participants in the ivermectin and control arms will receive products that differ in aspect and numbers. Participants will be informed that they are taking one of two antihelminthics. The sponsor and the study statistician will remain blinded to the assignment of each cluster group. All unblinded DSMB (Data and Safety Monitoring Board) reports will be prepared by an independent statistician.
Why?
From the Protocol supplementary material in PDF (page 110/266):
Given the use of albendazole in the control group, fully blinding the study would require a double-dummy approach with placebo-ivermectin and placebo-albendazole tablets. Ivermectin is only available in 3 mg tablets and it is dosed by body weight. This results in a relative high pill-burden with up to 13 tablets for heavier adults (see table 1). Adding placebo pills would further increase this burden. It is for this reason that the study is open-label but assessor blinded.
Screening
Pregnancy tests were required prior to dosing to minimize the possibility of embryonic/fetal harm from a drug with an uncertain pregnancy risk. Participants also had their skin checked for signs of other parasitic diseases. An added benefit is that other parasitic diseases are expected to be decreased by this public health intervention. Treatment for malaria was administered right away, when any of the children tested positive for malaria, regardless of whether the participants had symptoms.
Dosing
A finger stick blood test was used to detect malaria and to check drug levels. The dose of ivermectin was larger than currently given in Kenya, but within limits approved for use in the Netherlands and Germany. The repeated dosing of ivermectin and albendazole are more frequent than recommended anywhere, but calculations of elimination rates were expected to decrease the amount of drug remaining in the blood, at the time of the next dose, to less than 1% of the maximum blood level by the time the next dose would be given. Toxicity was not expected to be a significant risk, but was screened for. The safety results of the study did not show problems that any reviewers attributed to the increased doses of the study drugs.
From page 110/266 of the Protocol supplementary material in PDF. Table 1.
This dosing table seems as if it is encouraging a higher dose than is reasonable, but the displayed numbers are only the highest dose for that weight range. The doses are all multiples of 3 mg tablets. The highest per kg dose listed is for the 31-40 kg participants, the dose is 484 µg per kg for the lightest patients in that weight range. These are the only weight group receiving 5 tablets (15 mg ivermectin). 15 mg ranges from 375 µg per kg for the 40 kg participants up to 484 µg per kg for the 31 kg participants. Breaking up tablets to get more precise dosing was probably considered impractical.
This was during the rainy season. Transportation was by motorbike and bicycle, so the niceties of a hospital lab, or even the niceties of a watertight vehicle, were not available at the time of dosing. All of the participants were weighed, apparently using portable scales. The scales were cleaned and placed on a flat surface, at the time of dosing for an accurate weight.
Controlling Contamination
Contamination: a state of heterogeneous or impure roll out of the intervention in any given cluster or group of clusters. This implies a risk of over- or under estimating the effect size. Given that the maximum recorded dispersal of Anophelines in East Africa is 661 meters (42), contamination risk will be assessed by calculating the mean proportion of households with equal assignment in a 1 km radius around every core household. For control households only ivermectin-treated households within this 1 km radius may cause contamination. For ivermectin-treated households both control and non-participating households may cause contamination (Figure 7).
Why do the researchers worry about contamination? If the intervention affects the health of the mosquitoes that bite participants in the ivermectin group, the ivermectin is expected to harm those mosquitoes. Less healthy mosquitoes are less likely to spread malaria to participants in the control group (participants receiving albendazole) and less likely to spread malaria to the non-participants. Non-participants are part of the calculation of the efficacy of the intervention, if the possibility of contamination is not controlled for.
Having non-participants, who benefited from the intervention, but are not part of the intervention group, included in the calculations benefit from the intervention makes the intervention appear less effective. Since the rate of malaria among the non-participants is calculated using all of the people counted in the cluster, there would be contamination of the results. The clusters are arranged with a buffer area to prevent that beneficial effect to non-participants from affecting the statistical results from contamination by that bias. There is no actual contamination of non-participants by ivermectin. Each participant takes several tablets to get their monthly dose. This dose would be much more than a mosquito could carry to the next person they bite, even if the mosquito bit the study participant when the study participant’s blood concentration of ivermectin was at its highest.
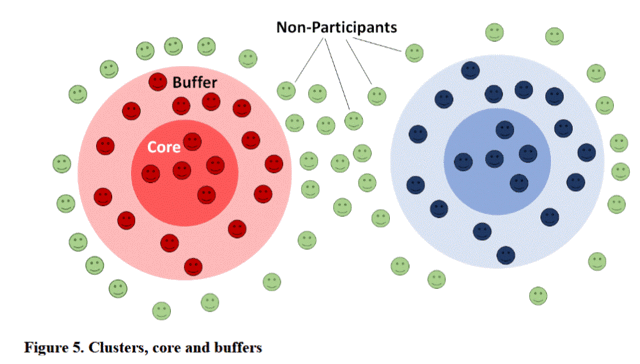
From page 120/266 of the Protocol supplementary material in PDF. Figure 5.
What were the results?
A total of 84 clusters comprising 28,932 eligible participants underwent randomization. The baseline characteristics of the participants were similar in the trial groups. Six months after the first round of treatment, the incidence of malaria infection was 2.20 per child-year at risk in the ivermectin group and 2.66 per child-year at risk in the albendazole group; the adjusted incidence rate ratio (ivermectin vs. albendazole) was 0.74 (95% confidence interval (CI), 0.58 to 0.95, P=0.02). The incidence of serious adverse events per 100 treatments did not differ significantly between the trial groups (incidence rate ratio, 0.63; 95% CI, 0.21 to 1.91).
26% lower incidence of malaria infection is not a huge percentage, but malaria kills a lot of children. A small percentage of a lot of people is still a lot of people. Yet, even saving the lives of a hundred children would be great. This public health intervention could save tens of thousands of lives and prevent millions of cases of malaria. Malaria is not the only medical condition that could be made less harmful with this intervention.
Mass administration of ivermectin would be a drug-based approach to vector control that leverages a mechanism of action that is distinct from that of the currently used insecticides in public health programs. This innovative method not only enhances vector control but provides potential collateral benefits in areas in which neglected tropical diseases, such as scabies and diseases caused by soil-transmitted helminths and filariae, are also endemic,37-39 particularly if existing delivery systems are leveraged for the distribution of ivermectin.40,41
This trial provides evidence to support the use of ivermectin as a complementary strategy for malaria control and prevention in areas where malaria is mesoendemic and transmission is perennial.
Why should we care?
Kenya is on the equator, so they do not have the kinds of seasons we are used to outside of the tropics, yet. Maybe this intervention will help to avoid making malaria endemic in America again. Currently, almost all cases are due to travel to places where malaria is endemic, but there have been occasional cases transmitted within the United States. Both malaria and yellow fever (caused by a virus, not a parasite, but also transmitted by mosquitoes) used to be right at home in the United States. Since these diseases are not foreign to the United States, the environment may support their return. A wall won’t keep mosquitoes out. Screwworms are another problem a wall won’t solve. The plan to stop the spread of screwworms by dropping sterile flies from aircraft in the Darién Gap in Panama is no longer working.
Public health measures are becoming more important, so we should be investing more in research on public health.
The trial was conducted in accordance with the principles of the Declaration of Helsinki, . . .
That is the beginning of a paragraph describing the ethical requirements the researchers met. The concern some might raise is that the active control group is not receiving an inert placebo. They are receiving an effective intervention. Depriving research participants of effective treatment is prohibited by ethical standards, such as the Declaration of Helsinki. People who understand science and ethics recognize that depriving research participants of a safe and effective intervention is causing harm. Causing harm to one group of research participants, for no expected benefit, is no longer allowed.
Science improves by learning from the mistakes of scientists. This is why the United States Public Health Service Study of Untreated Syphilis in the Negro Male at Tuskegee and Macon County, Alabama, 1932 -1972 is notorious. Research participants were deprived of a safe and effective intervention for no benefit to the research participants.
Starvation is at its lowest level due to advances made by scientists, including Fritz Haber, whose discovery enabled the large-scale production of synthetic fertilizers, feeding billions and preventing global famine. Then again, Haber also came up with a method for poisoning soldiers on the battlefield and another method for poisoning prisoners in extermination camps. Haber was not exactly altruistic, but he did save many times more lives than he helped to kill – and Haber was one of the most harmful scientists ever.
The Declaration of Helsinki and its predecessor the Nuremburg Code, were implemented to prevent those types of abuse.
Science – and scientists – save lives
To paraphrase the fictional Dr. Ian Malcolm, “Your anti-science propagandists were so preoccupied with whether or not they could spread Fear, Uncertainty, and Dread, they didn’t stop to think if they should.” That is not what is meant by people using the original quote, but it highlights the problem of pretending that competent scientists do not already study the safety of interventions in many ways before these interventions even leave a laboratory. But the safety of every intervention is studied at every step along the way, beginning with animal studies all the way up to final approval, and it continues to be monitored after approval.
The people usually quoting the mathematician from Jurassic Park would have us believe that these forms of preventive medicine are going to kill us all, because scientists are the problem. But opposition to science is the real problem.
Opposition to science doesn’t stop scientists from saving lives, but this opposition can decrease the benefits of science. Regulation is what helps people to do science well, even when it seems as if everything and everyone is trying to coerce scientists toward irresponsible behavior. Public health interventions treat people who are healthy, or not as sick as they could be without public health interventions. These public health interventions are intended to prevent harm, but the irrational human brain does a horrible job of assessing the relative risks of a possibly severe medical condition and a probably very safe medical intervention.









